What do you know about the chemical structure of phthalic anhydride (PA)? This material is known as a white organic compound that has a pungent odor. Phthalic anhydride can be dissolved in other organic solvents, water, alcohols. This material is used in the manufacture of plasticizers, phthaleins, resins, most emollients, and insecticides.
Today we see that the chemical structure of phthalic anhydride is made by naphthalene or catalytic oxidation of O-xylene. Another way to get phthalic anhydride is through phthalic acid. This material is an intermediate element in organic chemistry. Phthalic anhydride is readily available due to its dual function:
The first application of phthalic anhydride, or PA, is a chemical mediator for the production of polyvinyl chloride emollients.

Application of phthalic anhydride
Introduction to the chemical structure of phthalic anhydride (PA)
Phthalic anhydride, or PA, is a white, coarse-grained powder. It has a crystalline state and has the following chemical properties:
- Molecular weight: 148.12
- Molecular formula: C8H4o3
- Boiling point: 284.5
- Melting point: 131.1
- Spontaneous flashpoint: 584
- Solid density: 1.527 g/m3
- Enclosed are flash point 165, free ignition 151
- This material is soluble in most organic solvents, benzene, ether, and alcohol.
Applications of phthalic anhydride in various industries
The chemical structure of phthalic anhydride (PA) is primarily an intermediate chemical material. This material is used as a monomer in the construction of polymer structures. It is also widely used in the manufacture of UPR or unsaturated polyester resins.
This product is used in reinforced plastics for many applications such as the construction industry, electronics, car parts, manufacturing of bath equipment and yachts. Another significant application of this material is its use in the manufacture of alkyd resins.
These resins are also used in their place to make final products such as coatings, varnishes, and paints. Phthalic anhydride is used as a raw material for the manufacture of PVC phthalates, plasticizers, or softeners for normal use, and as a base material for the production of most chemicals, including saccharins and polyester polyethylenes. Another application of this material is its use in fire repellents, special paints, and pigments.
How to produce the chemical structure of phthalic anhydride (PA)
There are two methods for producing the chemical structure of phthalic anhydride (PA):
Laboratory production:
This method is performed for small scales using oxidizing devices by oxidation of naphthalene or 1,2-dimethylbenzene. Another way to obtain phthalic anhydride is to heat the phthalic anhydride; After decomposing into phthalic anhydride and water, it is sublimated at 300 ° C and then collected.
Industrial method:
This method has been used in the past to use air oxygen and the oxidation of naphthalene in the presence of a suitable catalyst such as vanadium pentoxide. But today, this material is produced in another way, which is done by using ortho-xylene oxidation with the use of a suitable catalyst and in the presence of oxygen in the air.
Regarding the production process of phthalic anhydride, first, in a concentrated sulfuric acid solution, naphthalene was oxidized in the presence of mercury sulfate. Over time, this pathway can be performed by using a vanadium oxide catalyst with the oxidation of the naphthalene catalytic vapor phase.
We now see that naphthalene raw materials are generally being replaced by orthoxylene. Currently, only 16% of raw materials are supplied by naphthalene.
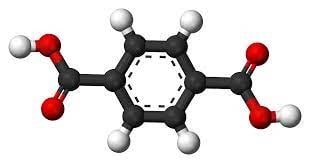
Structure of phthalic anhydride
Risks of using phthalic anhydride
The dust produced by this substance is quite irritating and can cause allergies to the respiratory system. The vapor from these materials can cause eye problems. Another important point about this substance is its rapid reaction to water. If its vapor is exposed to hot water, it reacts quickly and produces phthalic acid, which is quickly eliminated. Due to this, this material should be kept away from them.
Phthalic anhydride is currently produced by the oxidation of ortho-xylene or naphthalene in the gas phase in the presence of air currents, titanium dioxide, and vanadium pentoxide. This reaction is exothermic and is produced as a by-product of maleic anhydride.

